With the increasing investigation of DDR/PARP inhibitors, alone or in combination with chemotherapy or immunotherapy, there is a need for blood-based, robust, and repeatable DDR assays.
ANGLE has developed immunofluorescence assays to identify two DNA damage markers – phosphorylated histone variant H2AX (γH2AX) and phosphorylated KRAB-associated protein 1 (pKAP1) – in circulating tumour cells (CTCs) enriched using ANGLE’s Parsortix®system.
For use in the research setting as an endpoint in clinical studies, these assays make longitudinal, repeatable monitoring of treatment response possible. The process involves shipping patient blood samples to ANGLE’s GCP-compliant laboratory where the CTC enrichment and immunofluorescent detection are carried out, and a results report provided.
Assay performance
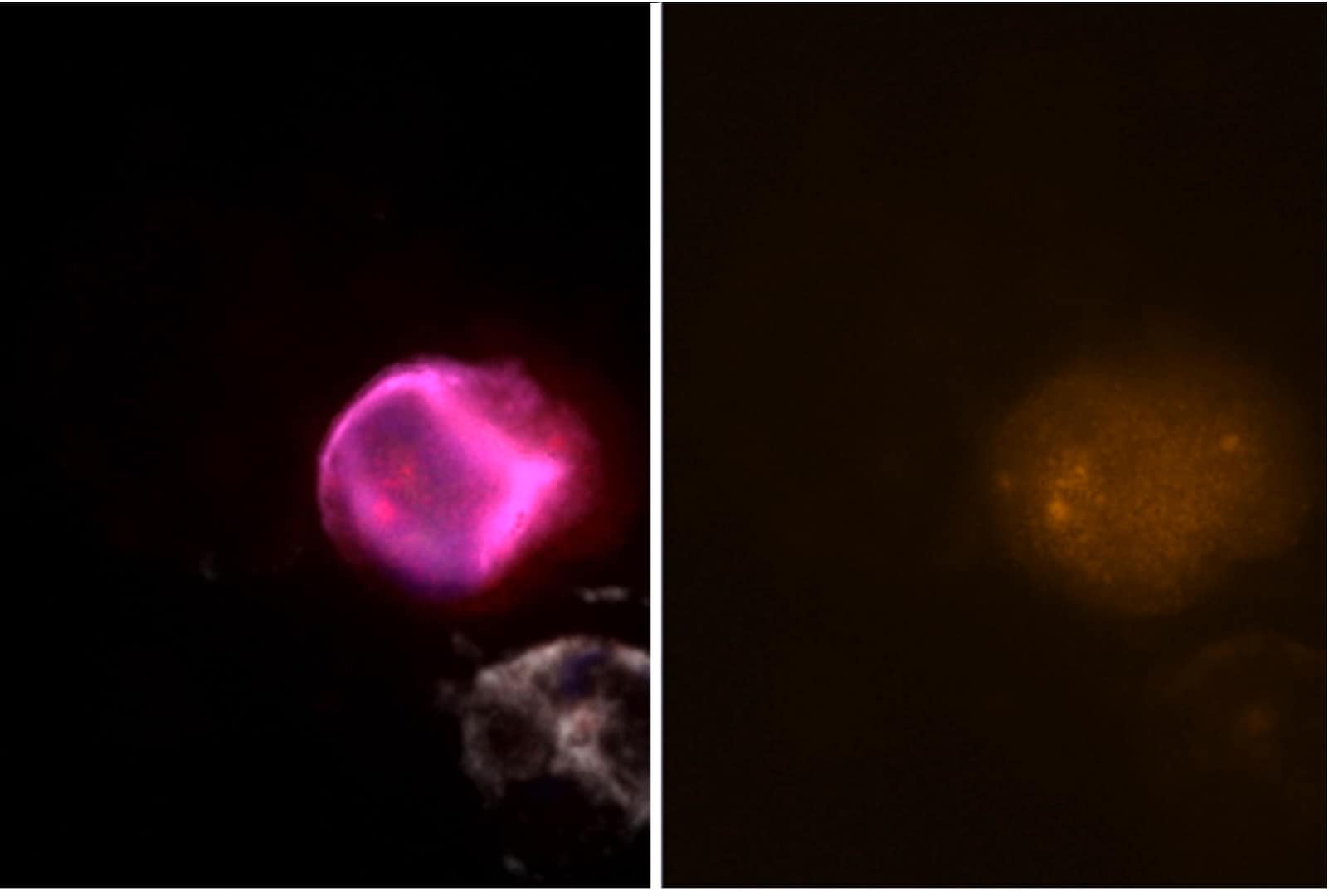
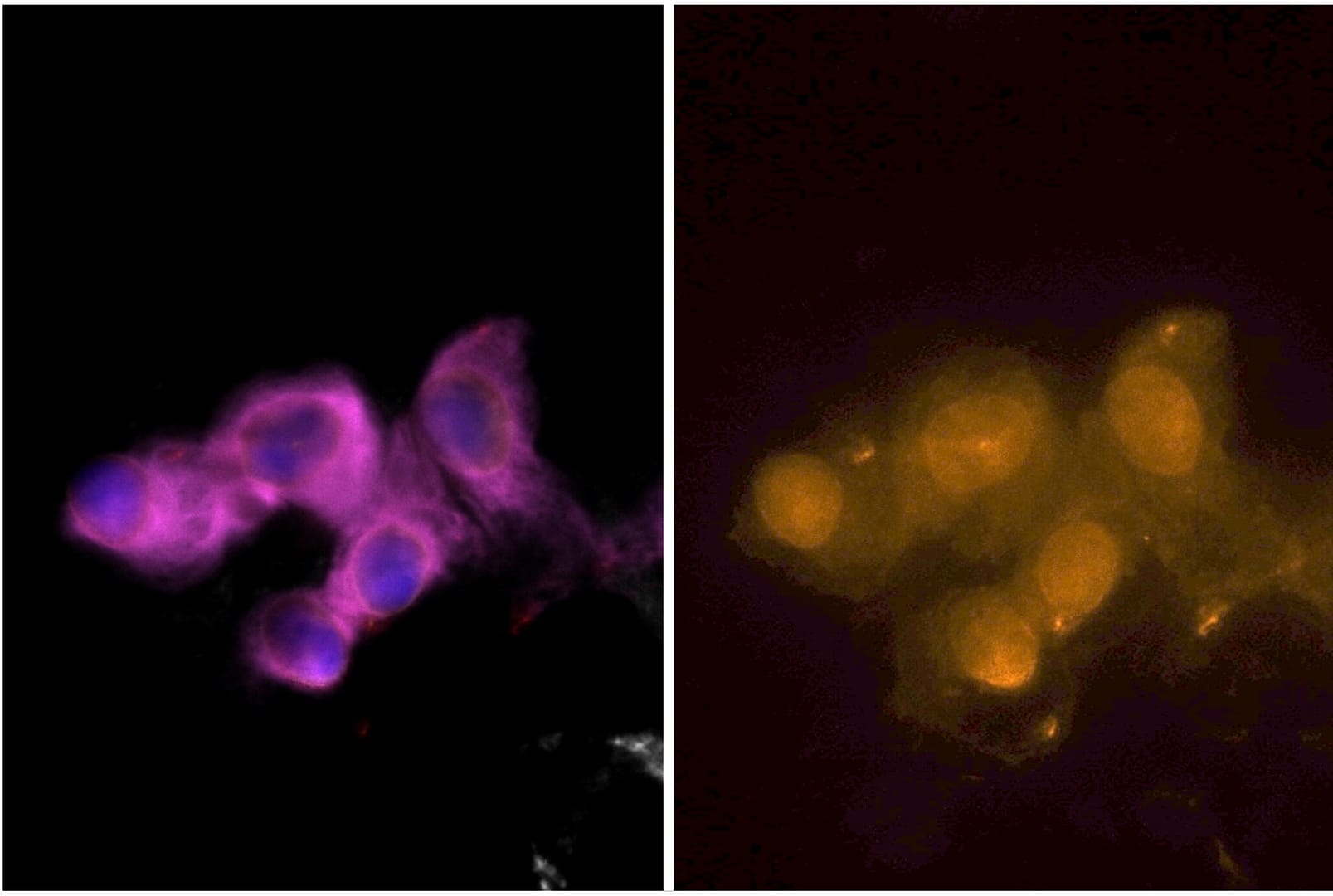
The assays have been fully evaluated and verified in cancer cell models and tested for feasibility in cancer patient samples. They demonstrate high analytical sensitivity and analytical specificity, with positive nuclear staining in epithelial and mesenchymal CTCs.
Mean performance metrics using etoposide-treated and untreated cancer cell lines (MCF7 – γH2AX; H226 – pKAP1) spiked into healthy volunteer blood and recovered using Parsortix technology.
γH2AX
87%
Analytical Sensitivity
γH2AX
>99%
Analytical Specificity
pKAP1
82%
Analytical Sensitivity
pKAP1
100%
Analytical Specificity
Sample staining
Assay performance
Dose response
Dose response of γH2AX or pKAP1 positivity in cancer cells (MCF7 – γH2AX; H226 – pKAP1) following treatment with increasing doses of etoposide.
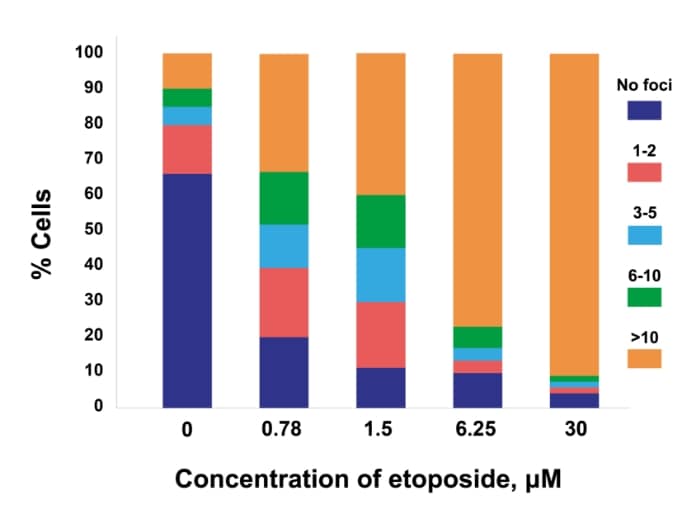
γH2AX
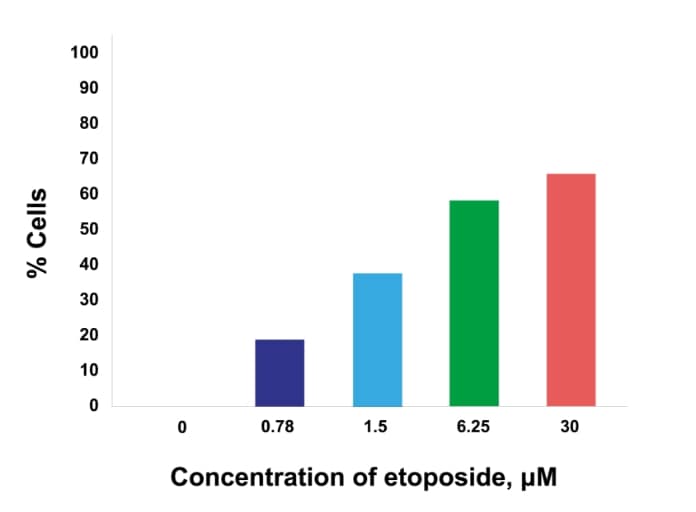
pKAP1
Linearity analysis
Increasing numbers of γH2AX-expressing MCF7 cells or pKAP1-expressing H226 cells were spiked into whole blood, recovered using Parsortix technology, harvested onto slides and stained using an epithelial/mesenchymal antibody panel and γH2AX or pKAP1.
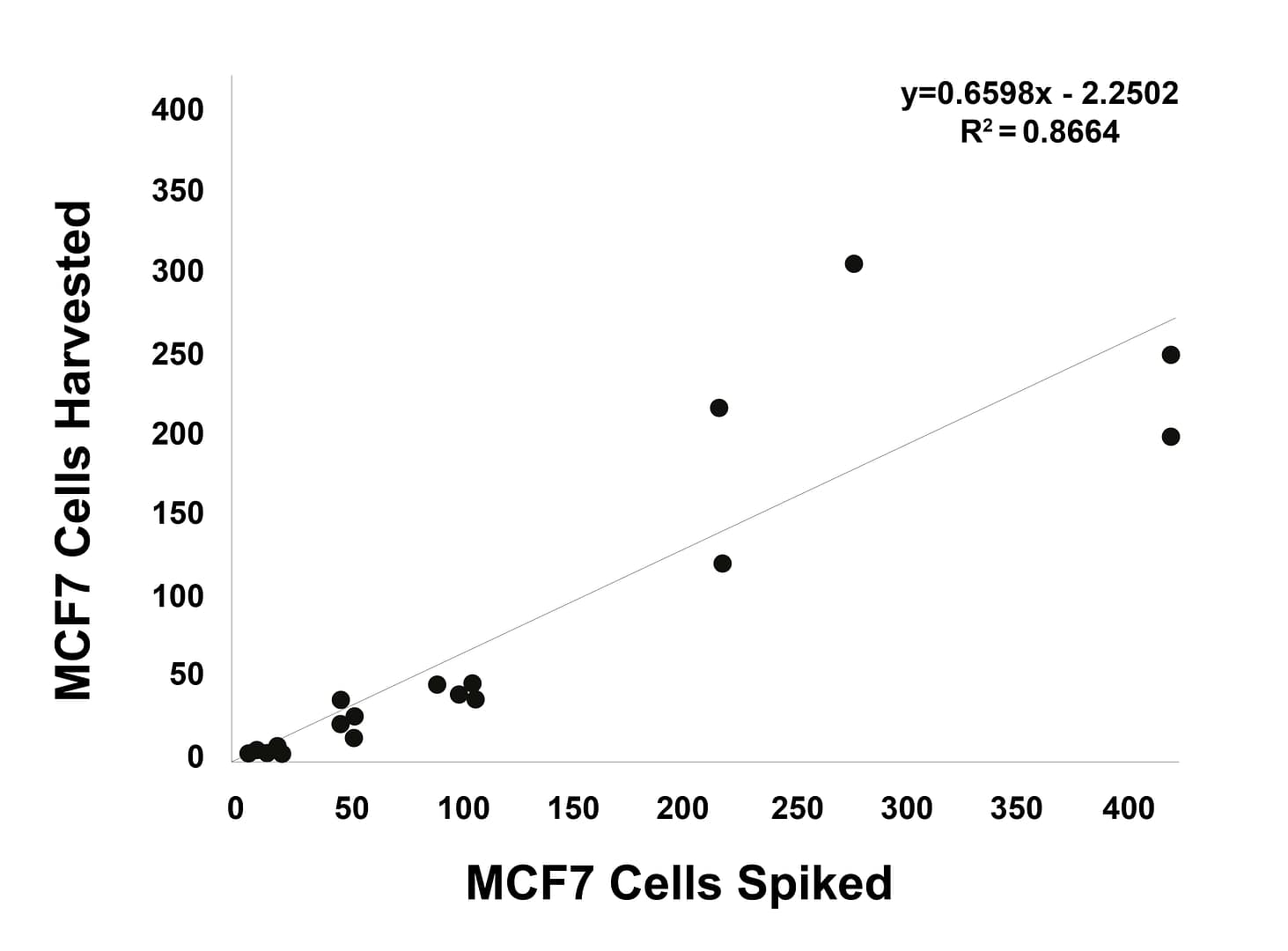
γH2AX
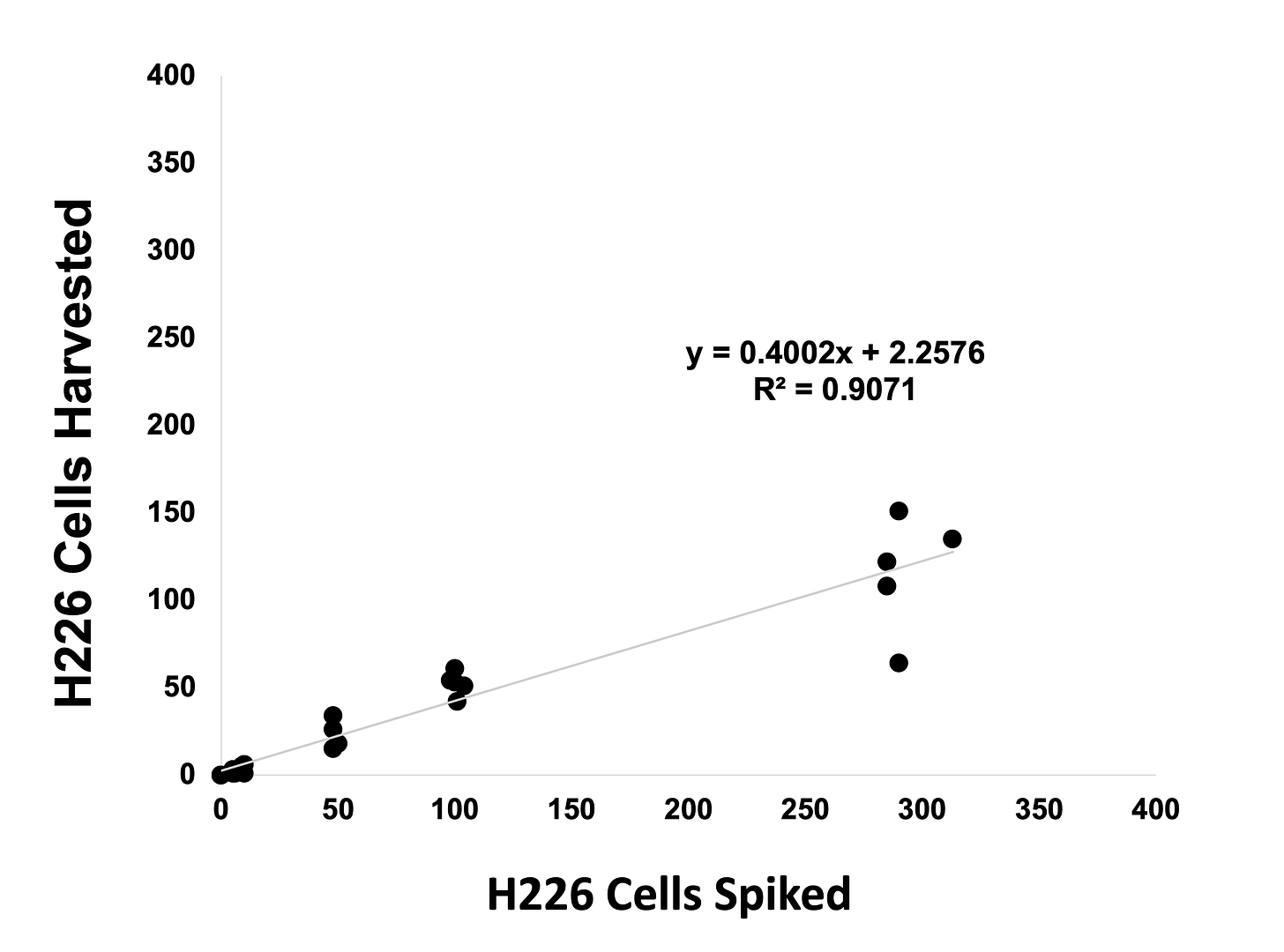
pKAP1
Clinical findings
The markers and assays are built on a foundation of robust evidence from clinical studies:
These studies highlight the utility of CTCs and DDR marker analysis for minimally invasive and rapid assessment of treatment response over time.
For Research Use Only. Not For Use in Diagnostic Procedures.
Resources related to DNA damage response (DDR)
Posters
January 2025
DDRi Summit 2025: Monitoring of DNA Damage Response Biomarkers using Circulating Tumour Cells captured with ANGLE’s Parsortix® instrument from Ovarian, Prostate, and Breast cancer patients
ANGLE Europe Limited, Guildford, UK, published at the 8th Annual DDR Inhibitors Summit 2025
Discover how DDR assays can benefit your trial
Leave your name and email address and we will be in touch with more information.
References
- Wang LH, Pfister TD, Parchment RE, et al. Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin Cancer Res. 2010;16(3):1073-84. doi: 10.1158/1078-0432.CCR-09-2799;
- Chatzkel J, Mocha J, Smith J, et al. Circulating tumor cells and γH2AX as biomarkers for responsiveness to radium-223 in advanced prostate cancer patients. Future Sci OA. 2019;6(1):FSO437. doi: 10.2144/fsoa-2019-0092;
- Tan AR, Chan N, Kiesel BF, et al. A phase I study of veliparib with cyclophosphamide and veliparib combined with doxorubicin and cyclophosphamide in advanced malignancies. Cancer Chemother Pharmacol. 2022;89(1):49-58. doi: 10.1007/s00280-021-04350-x