Portrait Flex takes circulating tumour cell (CTC) analysis to the next level.
Portrait Flex is an immunofluorescence (IF) assay for the identification of epithelial, mesenchymal and epithelial-to-mesenchymal transition (EMT) CTCs, plus an optional additional biomarker developed specifically for your study needs.
CTCs are first harvested from whole blood using ANGLE’s proprietary Parsortix® technology, which captures cells according to size and deformability. This method enables the effective isolation and enrichment of epithelial, mesenchymal, and epithelial-to-mesenchymal transition CTCs, as well as CTC clusters.
The Portrait Flex assay is then used to enumerate and characterise the CTCs via immunofluorescent (IF) staining for epithelial (FITC), mesenchymal (Cy7), blood lineage (Cy5), and nuclear (DAPI) markers.
Portrait Flex also offers an additional open channel that can be used to detect your therapeutic target or biomarker of interest. Examples of CTC biomarkers that can be placed into this open channel are HER2 and PD-L1.1–7
Why use Portrait Flex?
The assay…
- Enhance your clinical trial evaluations with valuable data points that can be used for patient selection, CTC enumeration and drug target biomarker identification.
- Detect your specific biomarker of interest, with a customisable open channel.
- Facilitate your patient selection and monitoring through improved CTC identification and analysis, due to optimised, epitope-independent CTC recovery.
The service…
- Have confidence that you will receive exceptional quality data thanks to ANGLE’s extensive experience in CTC capture, enumeration and characterisation.
- Experience global clinical trial support from ANGLE’s UK-based laboratory, ensuring flexibility to align with your trial schedule.
- Enhance the efficiency of your trial by streamlining patient selection to facilitate reduced trial size, costs and time with ANGLE’s end-to-end CTC solutions.
Portrait Flex analytical sensitivity and analytical specificity*
Key performance metrics using Hs 578T (mesenchymal) and SKBR3 (epithelial) cell lines spiked and recovered from peripheral blood using Parsortix technology. Mean analytical sensitivity and analytical specificity.
Epithelial
99.1%
Analytical Sensitivity
Epithelial
95.8%
Analytical Specificity
Mesenchymal
93.5%
Analytical Sensitivity
Mesenchymal
100%
Analytical Specificity
Example of Portrait Flex stained samples
Contrived samples showing cell lines a. SKBR3 (epithelial marker [FITC]) and b. Hs 578T (mesenchymal marker [Cy7]).
FITC = epithelial markers, Cy5 = blood lineage markers, Cy7 = mesenchymal markers, DAPI = nucleus

a

b
Patient sample results
- Samples from 8 Non-Small Cell Lung Cancer patients were processed using ANGLE’s Parsortix PC1 system and Portrait Flex IF assay workflow.
- CTCs were identified in 75% of the patients, with 33% of the CTC-positive patients having ≥ 1 CTC with PD-L1 overexpression.
- 100% of the patients with CTCs had ≥ 1 mesenchymal CTC.
- 80% of CTC-positive patients had ≥ 1 CTC cluster, ranging from 2 to 26 CTCs.
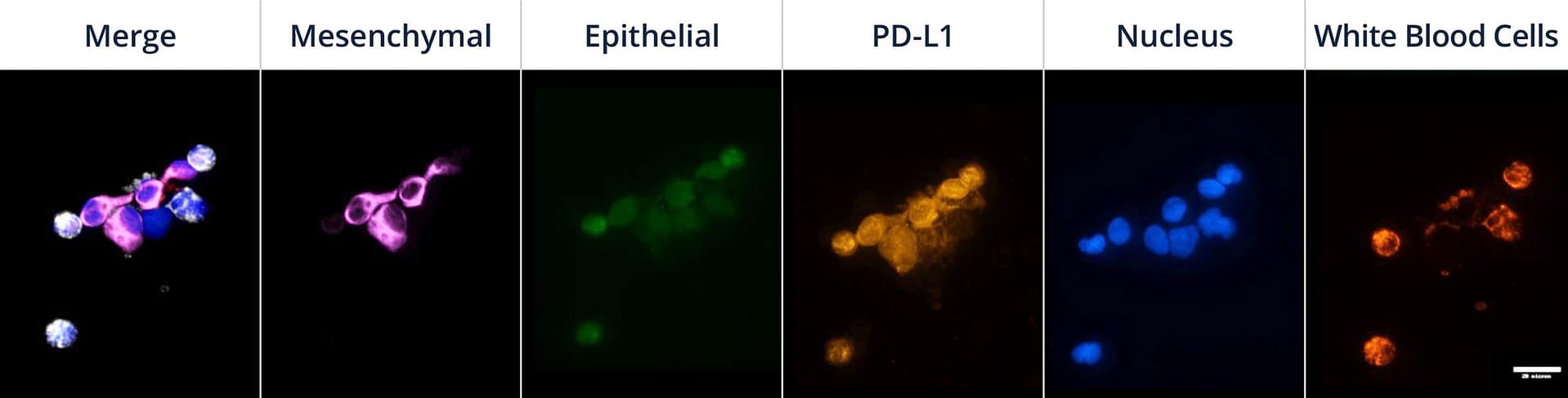
Mesenchymal CTCs isolated using Parsortix technology from a lung cancer patient and stained with the Portrait Flex assay with additional PD-L1 marker. CTCs show PD-L1 expression (purple = mesenchymal markers, green= epithelial markers, orange = PD-L 1, blue = nucleus, and red = blood lineage markers (white in merged image)).
For Research Use Only. Not For Use in Diagnostic Procedures.
Interested in knowing how Portrait Flex can help with your clinical trial testing? Leave your name and email address and we will be in touch with more information.
*Analytical sensitivity = proportion of spiked cells known to express the marker(s) of interest which were marker positive in the assay. Analytical specificity = proportion of spiked cells known to NOT express the marker(s) of interest which were marker negative in the assay.
References
1. Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 13, 395–412 (2018). 2. Silvestri, M. et al. Copy number alterations analysis of primary tumor tissue and circulating tumor cells from patients with early-stage triple negative breast cancer. Sci. Rep. 12, 1470 (2022). 3. Payne, K. et al. Characterizing the epithelial–mesenchymal transition status of circulating tumor cells in head and neck squamous cell carcinoma. Head Neck 44, 2545–2554 (2022). 4. Roche, J. The Epithelialto-Mesenchymal Transition in Cancer. Cancers 10, 52 (2018). 5. Cohen, E. N. et al. A Multi-Center Clinical Study to Harvest and Characterize Circulating Tumor Cells from Patients with Metastatic Breast Cancer Using the Parsortix®PC1 System. Cancers 14, 5238 (2022). 6. Reinhardt, F. et al. Diagnostic Leukapheresis Enables Reliable Transcriptomic Profiling of Single Circulating Tumor Cells to Characterize Inter-Cellular Heterogeneity in Terms of Endocrine Resistance. Cancers 11, (2019). 7. Borreguero-Munoz N, et al. Evaluation of HER2 status in circulating tumour cells isolated using ANGLE’s Parsortix System. Poster presented at AACR 2023 #1033. Cancer Res. 83(7_supplement), 1033 (2023).
